Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
MRSA Induced Septic Embolism in an Intravenous Drug User: How to Diagnose and Manage Septic Embolism
*Corresponding author:Leonard Ranasinghe, MD, California Northstate University College of Medicine, 9700 W. Taron Dr., ElkGrove, California, 95757, USA.
Received: June 18, 2022; Published: June 28, 2022
DOI: 10.34297/AJBSR.2022.16.002260
Abstract
A septic embolism occurs when an underlying infection causes a thrombus in a blood vessel to embolize and occlude a different blood vessel in the body. Since the embolus can travel to many different organs and cause ischemia, it can present with different symptoms and can cause life-threatening complications such as a pulmonary embolism. Due to this, it is essential to quickly recognize septic embolism in the Emergency Department and effectively treat the patient. This case report discusses a 47-year-old intravenous drug user male patient who presented with a constant sharp mid-upper back pain, tachycardia, and a heart murmur. This patient was started on IV antibiotics and imaged with a cardiac echo and computed tomography (CT) scan. Eventually, the diagnosis of Methicillin Resistant Staphylococcus aureus (MRSA) septic embolism was confirmed, and the patient was treated effectively with vancomycin. As there are multiple different diagnoses that present in a similar way, such as aortic dissection or bacterial endocarditis, looking at associated symptoms and using imaging can be useful to developing the final diagnosis. CT scans and positron emission tomography (PET) imaging are commonly used imaging techniques that are used to support and confirm the diagnosis of a septic embolism. Effective treatment involves the use of antibiotics (vancomycin, linezolid, and daptomycin) to treat the underlying MRSA infection and supportive care for any additional symptoms that the patient is experiencing. Leaving a patient undiagnosed with a septic embolism is associated with numerous additional complications; thus, it is necessary to quickly diagnose and treat this condition. A thorough examination of this case study will give a unique presentation of MRSA induced septic embolism in hopes of identifying and treating patients with this diagnosis.
Keywords: Septic embolism; Intravenous Drug User; Mid-upper back pain; Vancomycin
Abbreviations: MRSA: Methicillin Resistant Staphylococcus aureus; IV: Intravenous; CT: computed tomography; PET: positron emission tomography; DVT: deep vein thrombosis; PE: pulmonary embolism
Introduction
Septic embolism is a life-threatening complication of an infection involving the formation of a thrombus that occludes a crucial blood vessel and can result in infarction. It may lead to further complications of inflammation, infection and abscess formation [1]. The clots formed from septic emboli originate from the growth of bacterial colonies or inoculum; therefore, risk factors are those that create vulnerable environments for localized bacterial growth. These risk factors include implants, catheters, prosthetic bones, cardiovascular devices, intravenous drug use, surgeries, and any circumstance that introduces bacteria to the bloodstream [2]. The demographic distribution of septic embolism is not well studied. Since it is a complication that occurs due to circumstantial rather than genetic or social factors, there has not been any evidence suggesting an increased risk within a particular social group. However, environmental factors may be of consideration due to a correlation to increased exposure to certain pathogens associated with septic emboli.
Since septic emboli are caused by infections, various microorganisms have been identified as contributing to the pathogenesis of septic emboli. These include but are not limited to: Staphylococcus aureus, Streptococcus, Candida, Aspergillus, and other coagulase-negative Staphylococcus species [1]. These bacteria infect various sites in the body, including foreign objects such as cardiovascular devices or native structures such as heart valves. Upon forming a vegetation, the bacterial colony dislodges into the bloodstream, where it may fragment or remain intact. In the bloodstream, the vegetation or fragments of the vegetation lodge into vascular structures. At this point in the pathogenesis, the vegetation is termed a septic emboli and results in a twofold effect, termed a “double hit” injury [1]. The primary effect of the septic emboli arises from the effects of vaso-occlusion and resulting ischemia; this is often complicated by a secondary effect of increased inflammation and infection. This inflammation and infection can further promote thrombus formation. In fact, pathogens can cause septic emboli without forming clots but rather through toxins that mediate thrombus formation [3].
Regardless of pathogenesis, septic emboli manifest with symptoms and signs that vary widely depending on the location of the clot. Organs that can be affected by septic embolisms include the brain, the spleen, coronary arteries, mesenteric arteries, and multiple other structures [1]. Septic embolism typically causes chest pain, fevers, and difficulty breathing, if they occur in the heart or lungs.4 One of the most common bacterial agents that cause septic embolism is Methicillin Resistant Staphylococcus aureus (MRSA), which can cause bilateral lower extremity weakness and numerous nodules in lungs [5]. MRSA can enter the pulmonary vasculature with the venous return to the right side of the heart, where the microorganism has time to settle within the lung tissue. These symptoms appear due to thrombus causing an inflammatory reaction and causing parenchymal destruction. Once the pulmonary emboli are within the pulmonary tissue, they will lead to chest discomfort and shortness of breath due to a reduction in oxygen exchange.
Chest CT scan is the primary imaging modality to diagnose pulmonary emboli, which show nodules, pleural effusion, cavitation, and infiltrates that have been lodged into the lungs [6]. Due to the body’s immune response against MRSA, the patient can have a slightly elevated white blood cell count on their laboratory tests. Pulmonary nodules that are between 0.5 and 3.5 cm are indicative of septic emboli, specifically emboli caused by MRSA [7]. Blood cultures are helpful to isolate and identify the causative bacterial agents. However, negative blood cultures do not necessarily rule out a bacterial infection.
It is crucial to diagnose and treat patients with septic emboli in an efficient, effective, and timely manner because untreated septic embolisms may cause significant complications, including possible life- threatening conditions, in the patient. A rare, yet fatal, complication of MRSA septic embolism is the formation of a deep vein thrombosis (DVT) or a pulmonary embolism (PE) [8]. Both of these occur when a clot in the vasculature embolizes, travels through the circulation, and gets lodged in either veins of the legs or the small vasculature of the lungs, or both [9]. Both DVTs and PEs are more frequently seen in the elderly, however, it may also occur in young patients suffering from septic embolisms [10]. Additional complications that may occur due to untreated, progressive MRSA septic embolism include cavernous sinus thrombosis and its associated symptoms, brain abscesses, acute ischemic infarcts, meningitis, osteomyelitis, pituitary gland compression, necrotizing pneumonia, and vision loss from optic nerve compression [11]. Risk factors for developing the complications of MRSA septic embolism include musculoskeletal infection, endovascular infection, being of African American descent, and delayed intervention because these are associated with a longer duration of MRSA bacteremia [12].
Since delayed intervention is associated with increased risk of life-threatening complications of septic embolisms, it is crucial for physicians to treat these patients early and rapidly. Linezolid has been commonly used in the treatment of MRSA septic embolisms; however, daptomycin may also be used for salvage therapies in a patient suffering from MRSA septic embolism associated infectious endocarditis and pulmonary complications [13]. The current state of literature agrees that linezolid and daptomycin are the treatment of choice since this strain of Staphylococcus aureus is resistant to many other types of commonly prescribed antibiotics and has the ability to develop resistance to antibiotics [14]. Even in the case of complications that arise from MRSA septic embolism, linezolid is an excellent choice to treat the underlying infection and use other supportive treatments to address any additional symptoms that the patient may be experiencing [10]. Since this condition is more likely in patients who are intravenous (IV) drug users, abstaining from IV drug use will result in a decreased probability of this condition [15]. Although MRSA septic embolism is treatable, it can lead to severe complications if undiagnosed and untreated; a review of the following case will summarize a potential symptom and laboratory sequelae that can lead to a MRSA septic embolism diagnosis and how to treat the patient [16].
Materials and Methods
A review of a clinical case was conducted to demonstrate a reallife example of septic embolism. The clinical case was compiled by Dr. Nalin Ranasinghe and the ED staff at Northeastern Hospital and was authorized by the proper consent forms signed by the patient.
Case Presentation
A 47-year-old male who had Hepatitis C and was an intravenous drug user presented to the Emergency Department with the chief complaint of mid-upper back pain. He stated that the symptoms began three days ago and described the pain as a constant sharp pain that was worse with movement of his torso. He also mentioned that he had bilateral pain on his feet and ankles that progressed up his body six days ago; his feet and ankle no longer have pain. The patient reports nausea without vomiting, decreased appetite (but can tolerate liquids), and occasional chills. The patient has chronic bilateral lower extremity edema which he claims to be his baseline. He reports that he smokes less than a half pack of cigarettes per day and last used IV drugs yesterday at noon. He mentions that he occasionally reuses the needles up to two times and has recently been injecting into the veins of his hands. The patient’s vital signs were BP: 112/77 HR: 86 RR: 18 Temperature: 37.1 °C O2 sat: 99% BMI: 25.80 kg/m2.
On physical examination, the patient was not in acute distress. He was tachycardic and a systolic murmur was heard, causing concern for endocarditis. Both right and left-lower lung fields revealed rales. Both his right and left lower legs had 1+ pitting edema present. Blood tests showed an elevated white blood cell count with bandemia, an elevated alkaline phosphatase, and mildly elevated B-type natriuretic peptide (BNP). The patient was started on IV antibiotics, an echo was done to rule out endocarditis and bacteremia was ruled out. A computed tomography (CT) scan of his chest was done and revealed findings concerning for a bilateral septic embolus.
The patient returned exactly one week later stating that he still had mid-upper back pain, chills, and nausea. The blood cultures taken last week were positive for Methicillin Resistant Staphylococcus aureus (MRSA), but when the patient’s home was called yesterday, he did not pick up and states that he never got the call. In the review of systems, the patient has chills and fevers, nausea and vomiting, chest pain, back pain, skin wound, and a dysphoric mood. The patient’s vital signs were BP: 113/73 HR: 109 RR: 22 Temperature: 37.4 °C O2 sat: 94%. On physical exam, the patient was ill-appearing, tachycardic, and had extra systolic heart murmurs. Both of his lower legs had 2+ edema and his left lower leg had a laceration. His capillary refill time was 2-3 seconds. One blood lab, the patient had a high white blood cell count of 23.2*103 cells/μL, low hemoglobin and hematocrit of 9.6 g/dL and 27.8% respectively, low sodium, chloride, and calcium at 126 mmol/L, 92 mmol/L, and 8.4 mg/dL respectively, high BUN/Creatinine ratio of 27, high total bilirubin of 1.7 mg/dL, and high AST of 152 U/L.
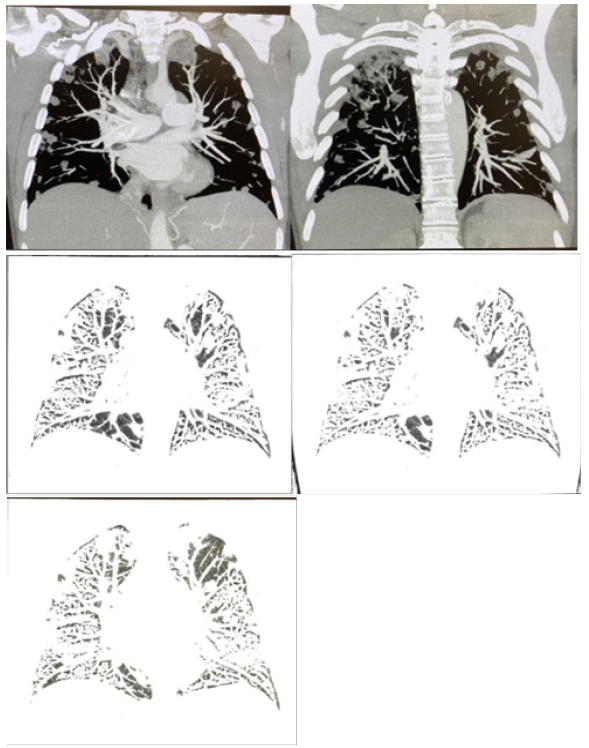
The patient was imaged with a chest CT with IV contrast as shown by Figure 1 and compared to his imaging from last week. He had a dilation of 4.0 cm of the main pulmonary artery and trace pericardial effusion. Imaging also found innumerable new predominantly peripheral cavitary nodules throughout both lungs, multiple enlarged mediastinal and hilar nodes, 4 cm cyst on the left kidney and mild degenerative changes of the thoracic spine.
Results and Discussion
Differential Diagnosis
With a chief complaint of mid-back pain, there are many different pathologies that the patient could be experiencing. A common cause of this is acute cholecystitis or obstruction of the common bile duct, however, CT imaging ruled out this cause [17]. Since the patient is an intravenous drug user, his symptoms could be caused by a drug overdose or drug dependency. The patient lacked common signs of overdose or dependency such as psychoticism, paranoia, miosis, and respiratory depression, thus making this diagnosis unlikely [18].
Other causes of the patient’s presentation could be pneumonia, however, the patient did not report shortness of breath or stabbing chest pain on inspiration [19]. The complaints of chest pain in the patient could also be a sign of pulmonary hypertension, in which the pressure in the pulmonary arteries is higher than normal leading to possible shortness of breath or pain within the chest 3 [20]. Since the patient did not present with dyspnea, pulmonary hypertension would be an unlikely diagnosis in this case.
The addition of tachycardia and a systolic heart murmur suggests there may be involvement of the heart directly. An example would be aortic dissection, which presents as severe chest and back pain that radiates to the neck or to the back [21]. Other common symptoms of an aortic dissection include stomach pain, loss of consciousness, and weak pulse in one arm or leg; however, these are not present in the patient. Another potential cause of the cardiac physical examination findings in the patient is bacterial endocarditis caused by bacteremia. Although MRSA was cultured from the patient’s blood, a cardiac echo was done to rule out bacterial endocarditis [22]. The results of the chest CT scan supported a diagnosis of septic pulmonary embolism caused by MRSA, which is consistent with the presentation of the patient.
Imaging
Septic embolism is highly correlated with findings on CT and positron emission tomography (PET) imaging. A retrospective analysis of 20 patients found that there were bilateral pulmonary abnormalities in 90% of patients who had septic embolism, including: parenchymal opacities, nodular infiltrates, pleural effusion, and lymphadenopathy [23]. These clinical findings were present in CT imaging of the patient, specifically cavitary nodules, pulmonary artery dilation, trace pericardial effusion, and enlarged mediastinal and hilar lymph nodes.
Additionally, imaging of other body systems has been found to be a useful diagnostic tool and influential factor in treatment of septic embolism. Imaging of organs highly involved in the pathogenesis of septic embolism, such as the spleen, can be a confirmatory indication of the etiology [24]. Further, it has been found that spinal involvement of causative pathogens for septic embolism can change treatment priorities and objectives; in particular, a possible finding of spondylodiscitis may influence post interventional therapies [24]. Studies on the spine of our patient demonstrated mild degenerative changes as shown by Figure 1. Fortunately, these findings were not adequate to warrant concern. Nonetheless, imaging of the patient was an essential component of the diagnostic process that led to confirmation of septic embolism as well as produced findings useful in the management of the complication.
Management
Treatment of a patient with septic embolism includes two main tasks: treat the underlying infection and treat the symptoms. In our case, the patient’s blood culture showed MRSA infection; thus, he was given IV antibiotics that included vancomycin. This is an effective antibiotic to eradicate MRSA from the body. Other antibiotics that have been proven to work for MRSA septic embolism are daptomycin and linezolid [13]. In addition to antibiotics, some patients would benefit from drainage of the extrapulmonary infection if the infection has spread profoundly [25]. Due to the embolism, another possible treatment in addition to the antibiotics is aspiration thrombectomy followed by balloon angioplasty [26].
If the infection was not MRSA, then use laboratory tests to identify the causative agent and which drugs the agent is susceptible to. Common causes of septic embolism that are not due to MRSA are Klebsiella pneumoniae and Viridans streptococci [25]. For these cases, effective treatment includes antibiotics such as third generation cephalosporin and vancomycin as well as drainage and ultrasound-guided aspiration [27].
Conclusion
The patient presented to the Emergency Department with midupper back pain. Although this is a common presenting complaint in patients, it is crucial to consider the potential causes of the patient’s pain that may be life threatening. In this case, the patient’s history indicated intravenous drug use and his physical examination revealed heart murmurs. Blood cultures and a CT scan with contrast led to the diagnosis of MRSA induced septic embolism. This can be a difficult diagnosis to make due to the vague symptoms, however, it is important to consider the patient’s history and consider this complication of bacterial infection on the differential diagnosis. If septic embolism is not detected quickly, it can lead to catastrophic complications for the patient, including death. Definitive diagnosis can be made through CT or PET scans and the underlying infection should be treated with vancomycin, daptomycin, or linezolid, as well as supportive treatment as needed. With quick diagnosis and treatment, severe complications of MRSA induced septic embolism can be prevented.
Acknowledgment
The authors would like to thank Dr. Nalim Ranasinghe for providing the case and Dr. Leonard Ranasinghe for the continous mentorship throughout this project. The authors would also like to thank Dr. Arpita Vyas and Dr. Valeria Gerriets as well as California Northstate University for their ongoing support.
Conflict of Interest
There are no conflicts of interest to disclose.
References
- Elsaghir H, Al Khalili Y (2021) Septic Emboli. In: StatPearls. Treasure Island (FL).
- Stawicki S, Michael SF, Michael RL, Sarah BR, David CE, et al. (2013) Septic embolism in the intensive care unit. Int J Crit Illn Inj Sci 3(1): 58-63.
- Goswami U, Brenes JA, Punjabi GV, LeClaire MM, Williams DN (2014) Associations and Outcomes of Septic Pulmonary Embolism. TORMJ 8: 28-33.
- Rabinowitz DG, Stephen MC, Jeffrey IC, Jaclyn D, Robert NH, et al. (2020) MRSA septic pulmonary emboli presenting as isolated focal chest pain in an adolescent. Radiol Case Rep 15(11): 2406-2409.
- Christian Castillo, L. et al. (2019) Embolic Septic Emboli with MRSA: A different source. J Clin Intensive Care Med 4, 044-047.
- Ye R, Zhao L, Wang C, Wu X, Yan H (2014) Clinical characteristics of septic pulmonary embolism in adults: A systematic review. Respir Med 108(1): 1-8.
- Kuhlman JE, Fishman EK, Teigen C (1990) Pulmonary septic emboli: diagnosis with CT. Radiology 174(1): 211-213.
- Schaub RL, Rodkey ML (2012) Deep Vein Thrombosis and Septic Pulmonary Emboli with MRSA Osteomyelitis in a Pediatric Patient: Pediatr Emerg Care 28(9): 911-912.
- How J, Zhou A, Oh ST (2017) Splanchnic vein thrombosis in myeloproliferative neoplasms: pathophysiology and molecular mechanisms of disease. Ther Adv Hematol 8(3): 107-118.
- Mantadakis, Eleni P, Evridiki KV, Lambros M, Athanassios CE, et al. (2012) Deep venous thrombosis in children with musculoskeletal infections: the clinical evidence. Int J Infect Dis 16(4): e236-e243.
- Branson SV, McClintic E, Yeatts RP (2019) Septic Cavernous Sinus Thrombosis Associated with Orbital Cellulitis: A Report of 6 Cases and Review of Literature. Ophthalmic Plast Reconstr Surg 35(3): 272-280.
- Hamdy RF, Dona D, Jacobs MB, Gerber JS (2019) Risk Factors for Complications in Children with Staphylococcus aureus Bacteremia. J Pediatr 208: 214-220.
- Yazaki M, Oami T, Nakanishi K, Hase R, Watanabe H (2018) A successful salvage therapy with daptomycin and linezolid for right-sided infective endocarditis and septic pulmonary embolism caused by methicillin-resistant Staphylococcus aureus. J Infect Chemother 24(10): 845-848.
- Crass RL, Powell KL, Huang AM (2019) Daptomycin for the treatment of Staphylococcus aureus infections complicated by septic pulmonary emboli. Diagn Microbiol Infect Dis 93(2): 131-135.
- Cook RJ, Ashton RW, Aughenbaugh GL, Ryu JH (2005) Septic Pulmonary Embolism. Chest 128: 162-166.
- Twito J, Sahra S, Jahangir A, Mobarakai N (2021) A Curious Case of MRSA Bacteremia and Septic Pulmonary Embolism Secondary to Peripheral Venous Catheter. Case Rep Crit Care 2021: 1-4.
- Gillaspie DB, Davis KA, Schuster KM (2019) Total bilirubin trend as a predictor of common bile duct stones in acute cholecystitis and symptomatic cholelithiasis. Am J Surg 217(1): 98-102.
- Lugoboni F, Frances RL, Maria CP, Matteo M, Lorenzo Z, et al. (2017) Co-occurring Attention Deficit Hyperactivity Disorder symptoms in adults affected by heroin dependence: Patients characteristics and treatment needs. Psychiatry Res 250: 210-216.
- Marchello CS, Mark HE, Ariella PD, Eric TH, Ye Shen, et al. (2019) Signs and Symptoms That Rule out Community-Acquired Pneumonia in Outpatient Adults: A Systematic Review and Meta-Analysis. J Am Board Fam Med 32(2): 234-247.
- Hoeper MM, Hossein AG, Ekkehard G, Hans K, Horst O, et al. (2017) Pulmonary Hypertension. Dtsch Arztebl Int 114(5): 73-84.
- Liu Y, Maonan H, Jichun Z, Limei K, Yukui Ma, et al. (2020) Systematic Review and Meta-analysis of Current Literature on Isolated Abdominal Aortic Dissection. Eur J Vasc Endovasc Surg 59(4): 545-556.
- Shingu M, Naoto I, Jun Oh, Shimpei M, Yohei Kanzawa, et al. (2021) Hemolytic Anemia in a Patient with Subacute Bacterial Endocarditis by Cardiobacterium hominis. Intern Med 60(21): 3489-3495.
- Song XY, Shan Li, Jian Cao, Kai Xu, Hui Huang et al. (2016) Cardiac septic pulmonary embolism: A retrospective analysis of 20 cases in a Chinese population. Medicine 95(25): e3846.
- Amraoui S, Ghoufrane Tlili, Manav Sohal, Benjamin Berte, Elif Hindié, et al. (2016) Contribution of PET Imaging to the Diagnosis of Septic Embolism in Patients with Pacing Lead Endocarditis. JACC: Cardiovasc Imaging 9(3): 283-290.
- Lee SJ, Seung Ick Cha, Chang Ho Kim, Jae Yong Park, Tae Hoon Jung, et al. (2007) Septic pulmonary embolism in Korea: Microbiology, clinicoradiologic features, and treatment outcome. J Infect 54(3): 230-234.
- Maqsood K, Sarwar N, Eftekhari H, Lotfi A (2014) Septic Coronary Artery Embolism Treated with Aspiration Thrombectomy: Case Report and Review of Literature. Tex Heart Inst J 41(4): 437-439.
- Liu JW, Lin TC, Chang YT, Tsai CA, Hu SY (2017) Prostatic abscess of Klebsiella pneumonia complicating septic pulmonary emboli and meningitis: A case report and brief review. Asian Pac J Trop Med 10(1): 102-105.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.